
MiSight® 1 day
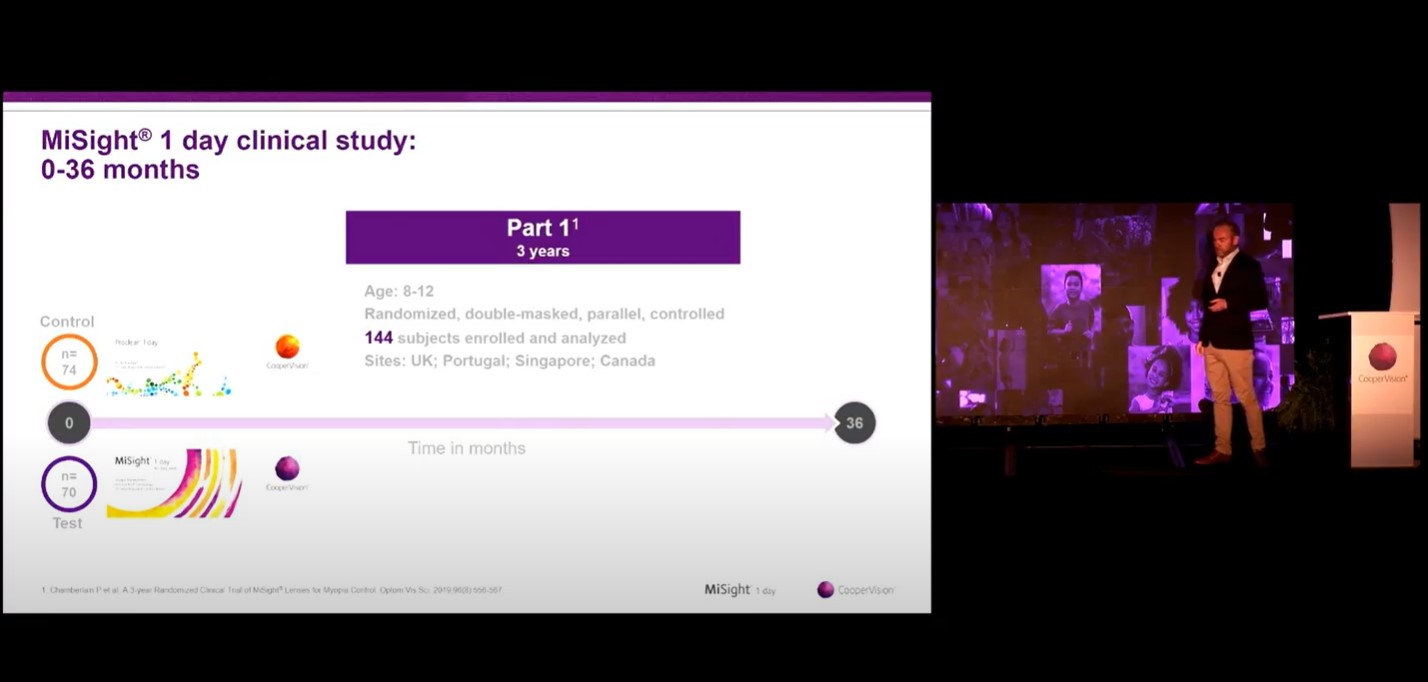
MiSight® 1 day is safe, well-tolerated, and has the longest and most robust data available on its safety and efficacy of any soft contact lens option for myopia control. It can be considered a first-line treatment for myopia correction and control. It is also the first and currently only myopia control intervention which has received United States FDA indication for myopia control. The MiSight® 1 day dual-focus contact lens has been demonstrated in two randomized clinical trials (two years' and three years' duration) to have a high safety profile, be effective, and well accepted by myopic children. It has the longest dataset available of any myopia controlling soft contact lens, with clinical study results published for up to six years and reported for up to seven years.
Visit the MiSight® 1 day website.
MiSight® 1 day Information
Product disclaimer
FDA Indications for use (US only): MiSight® 1 day (omafilcon A) soft (hydrophilic) contact lenses for daily wear are indicated for the correction of myopic ametropia and for slowing the progression of myopia in children with non-diseased eyes, who at the initiation of treatment are 8-12 years of age and have a refraction of -0.75 to -4.00 diopters (spherical equivalent) with ≤0.75 diopters of astigmatism. The lens is to be discarded after each removal.
Availability of interventions with regulatory indications to slow progression of myopia varies by country. CooperVision does not endorse off label prescribing of interventions for myopia control.
Articles on MiSight® 1 day.
CooperVision resources

New six year MiSight® 1 day research interview
In this interview, Paul Gifford and Kate Gifford of Myopia Profile chat to Arthur Bradley and Paul Chamberlain, CooperVision R&D leaders, about their newly published paper in Ophthalmic and Physiological Optics which explores a novel analysis on the cumulative treatment effect and efficacy of MiSight 1 day, from the six year clinical study.

One Thing - my myopia management journey
As part of Myopia Action Month in September 2023, CooperVision asked myopia experts around the world "What's the one thing that motivated you to start with myopia management?" Listen to leaders in the field discuss their journey into myopia management, and what they have learnt along the way.
The MiSight® 1 day Story
MiSight® 1 day contact lenses hold the largest body of evidence for childhood myopia management with soft contact lenses - listen to experts and researchers discuss the science behind MiSight® 1 day , and how this extensive and compelling research translates into practice.
